AZT, AIDS, Burroughs Wellcome, and Fauci: How a Failed Toxic Chemotherapy Drug Received Record Approval and Made Billions While Patients Paid the Ultimate Price.

The HIV/AIDS pandemic swept across the world in the 1980s. There is no known cure for HIV/AIDS. The fear permeating the United States and other nations during the AIDS pandemic pushed regulators and pharmaceutical companies to find a potential treatment. AZT, a failed chemotherapy drug that ultimately causes a reduction in T-4 cells, was approved faster than any drug in FDA history. AZT’s approval by the FDA and strong endorsement by the head of the National Institute of Health, Dr. Anthony Fauci, made Burrough Wellcome billions of dollars.
Approval was based on a single flawed test. AZT caused AIDS mutations and severe adverse reactions, including a reduction of red blood cells. AZT also forced patients to obtain regular blood transfusions. While AZT was marketed as a miracle drug based on a single flawed study and incomplete data, patients regularly endured severe adverse reactions and limited improvements.
Independent UK: The Rise and Fall of AZT
Key points from Independent UK’s story on the rise and fall of AZT:
- Prelimary study of AZT was published in The Lancet and was deadliest clinical trual run by the British Medical Research Council
- Individuals with HIV protested outside Terrance Higgins Trust (establoshed by Wellcome Trust) involving their endorsement of AZT
- AZT was developed to treat cancer and was used in chemotherapy
- AZT bypassed the typical 8-10 year FDA testing process in 20 months
- During the first human tests, AZT was shown to cross the blood-brain barrier
- AZT caused toxicity levels but was deemed safe
- During the second phase of testing, since fatality levels were far higher among the placebo group, individuals with AIDS were given AZT and trial was “unblinded” within two weeks
- AZT caused immediate but temporary spikes in T4 counts
- WHO declared over 13 million HIV/AIDS infections in the mid to late 1980s
- AZT was the only approved treatment for AIDS. As the number of individuals infected with AIDS increased, Wellcome’s stock prices increased dramatically
- Wellcome admitted AZT caused cancer in rodents but stated rodents were given 10 times the dose of AZT as humans
- Since the studies were unblinded and short (less than 6 months), the drop of T4 in AZT patients in the long-term was not discovered/included in data
- Wellcome donated money to AIDS organizations to produce positive informaiton about AZT. The Welcomme Trust popped up in the Fauci emails:
Fauci stated in the investigation by the USG, led Jeremy Farrar of the Wellcome Trust, would review the potential evolutionary origins of COVID-19, as that field of study was not Fauci’s “area of expertise.” The Wellcome Trust was created by Sir Henry Solomon Wellcome, who started a pharmaceutical company called Burroughs Wellcome & Co. in 1880.

Jeremy emailed Fauci on February 5th, 2020 for recommendations of individuals that would be willing to participate in a WHO group to “look at the origin and evolution of 2019n-CoV.” AaS of July 2021, Biden’s administration has determined the “lab leak” theory is credible. Fauci’s recommendations included Joseph DeRisi of the Chan Zuckerberg BioHub:


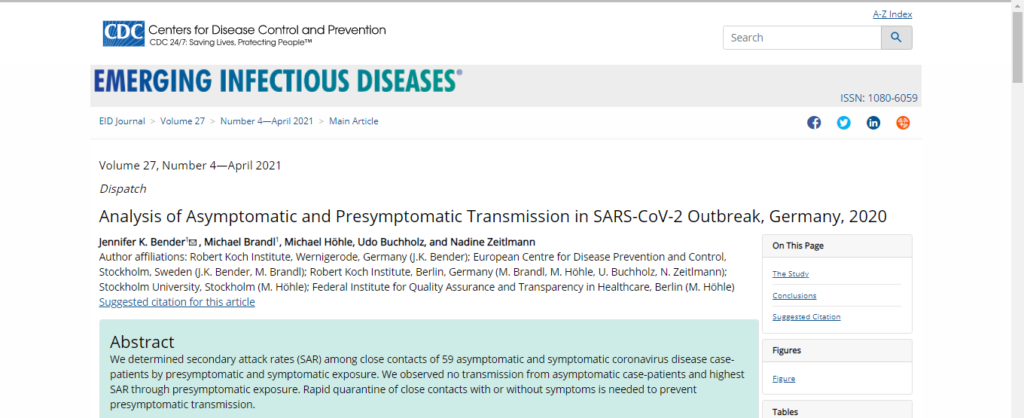
- The Lancet published a research paper that claimed that COVID-19 could be transmitted asymptomatically, which was contradicted by a study posted by the CDC:
“Asymptomatic carriers represent a significant risk for transmission. Containment of future outbreaks will depend on early testing in sectors and regions. Higher participation rates must be assured through targeted incentivisation and recurrent invitation”
Spin AZT Article
Spin wrote an extensive article about AZT in 1989, outlining issues with safety, flaws with studies, and corruption by regulators and Burroughs Wellcome:
“Undeniably, AZT kills T-4 cells [white blood cells vital to the immune system. No one can argue with that. AZT is a chain-terminating nucleotide, which means that it stops DNA replication. It seeks out any cell that is engaged in DNA replication and kills it. The place where most of this replication is taking place is in the bone marrow. That’s why the most common and severe side effect of the drug is bone marrow toxicity. That is why they [patients] need blood transfusions.”
Dr. Harvey Bialy, molecular biologist, former editor of Biotechnology
Key points from Spin AZT article:
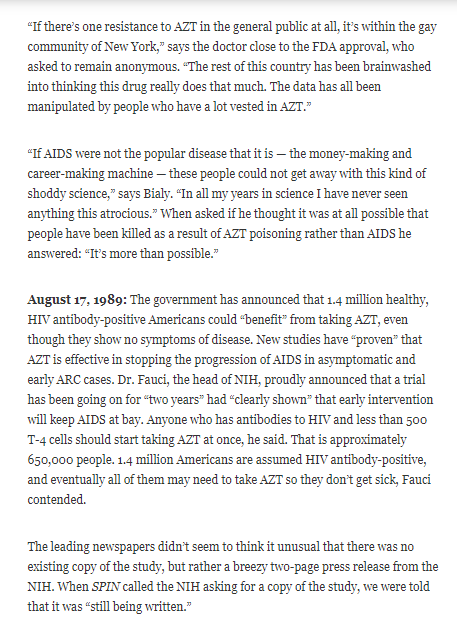
- “The doctors who had been consulted knew that the study was flawed and that the long-range effects were completely unknown. But the public was almost literally baying at the door. Understandably, there was immense pressure on the FDA to approve AZT, considering the climate of fear and anger all around.”
- Among an FDA panel consistening of 11 AIDS, only one member (Dr. Brook) voted against using AZT for AIDS and mentioned concerns with premature data, poorly compiled statistics, and evidence that AZT did not stop AIDS deaths
- Dr. Anthony Fauci supported the use of AZT and pushed to expand its use in August 1989
- Newspapers began reporting AZT was succeful in treating AIDS
- AZT was approved faster than any drug in FDA history and was the most expensive drug ever marketed ($8,000/annually excluding the cost of blood transfusions)
- “AZT is the only antiretroviral drug that has received FDA approval for treatment of AIDS since the epidemic began ten years ago, and the decision to approve it was based on a single study that has long been declared invalid. The study was intended to be a “double-blind placebo-controlled study,” the only kind of study that can effectively prove whether or not a drug works. In such a study, neither patient nor doctor is supposed to know if the patient is getting the drug or a placebo. In the case of AZT, the study became unblinded on all sides, after just a few weeks.”
- The only study used to support the approval of AZT was never completed
- Dr. Brook, who voted against FDA approval of AZT and warned that “Because of the most severe toxic effect of AZT — cell depletion of the bone marrow —patients would need frequent blood transfusions.”
- AZT “has been found to accelerate the very process it was said to prevent: the loss of T-4 cells.”
- Bone marrow suppression and depletion of red blood cells were among the 50 potential side effects Wellcome disclosed for AZT
- By 1989, all participants in the original AZT were dead
- Dr. Anthony Facui, head of the NIH claimed a study clearly showed AZT worked but the NIH was unable to provide a copy of the study to the media. The NIH was accussed of misrepresenting data, portraying AZT as more effective than it actually was
- A 1988 study conducted in France and published in The Lancet “that AZT was too toxic for most to tolerate, had no lasting effect on HIV blood levels, and left the patients with fewer T-4 cells than they started with. And that “the benefits of AZT are limited to a few months for ARC and AIDS patients. After a few months, the study found, AZT was completely ineffective.”
According to Time: